
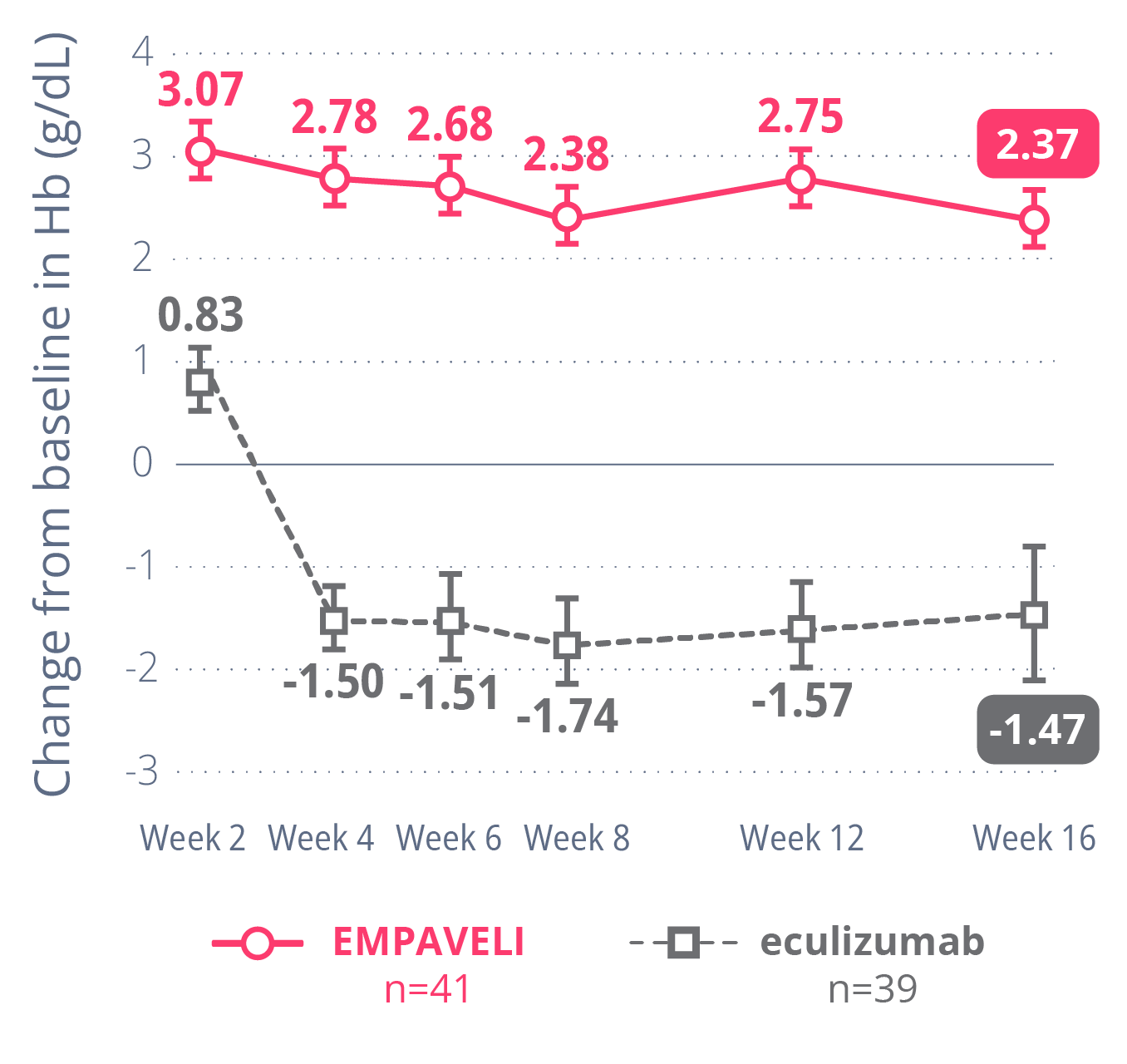
Hb DIFFERENCE
of 3.84 g/dL
with EMPAVELIcompared to eculizumab
(P<0.0001)
Average change in Hb levels from beginning to Week 16

Peg is an
adult who's taken
EMPAVELI.
EMPAVELI is a medicine that affects your immune system and may lower the ability of your immune system to fight infections.
EMPAVELI increases your chance of getting serious infections caused by encapsulated bacteria such as Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B. These serious infections may quickly become life-threatening or cause death if not recognized and treated early.
Your healthcare provider will give you a Patient Safety Card about the risk of serious infections. Carry it with you at all times during treatment and for 2 months after your last EMPAVELI dose. Your risk of serious infections may continue for several weeks after your last dose of EMPAVELI. It is important to show this card to any healthcare provider who treats you. This will help them diagnose and treat you quickly.
EMPAVELI is only available through a program called the EMPAVELI Risk Evaluation and Mitigation Strategy (REMS). Before you can take EMPAVELI, your healthcare provider must enroll in the EMPAVELI REMS program, counsel you about the risk of serious infections caused by certain bacteria, give you information about the symptoms of serious infections, make sure that you are vaccinated against serious infections caused by encapsulated bacteria and that you receive antibiotics if you need to start EMPAVELI right away and you are not up to date on your vaccines, and give you a Patient Safety Card about your risk of serious infections.
Do not take EMPAVELI if you:
Before you take EMPAVELI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the vaccines you receive and medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements which could affect your treatment.
If you stop taking EMPAVELI, your healthcare provider will need to monitor you closely for at least 8 weeks after stopping EMPAVELI. Stopping treatment with EMPAVELI may cause a breakdown of red blood cells due to PNH.
Symptoms or problems that can happen due to red blood cell breakdown include:
EMPAVELI can cause serious side effects including allergic reactions. Allergic reactions can happen during your EMPAVELI infusion. Stop your EMPAVELI infusion and tell your healthcare provider or get emergency medical care right away if you get any of these symptoms during your EMPAVELI infusion:
The most common side effects in people with PNH treated with EMPAVELI include injection-site reactions; infections; diarrhea; pain in the stomach (abdomen); respiratory tract infection; pain in the arms, hands, legs, or feet; low potassium in blood; tiredness; viral infection; cough; joint pain; dizziness; headache; and rash.
These are not all of the possible side effects of EMPAVELI. Tell your healthcare provider about any side effect that bothers you or that does not go away.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
EMPAVELI is a prescription medicine used to treat adults with a disease called paroxysmal nocturnal hemoglobinuria (PNH).
Please see full Prescribing Information, including Boxed WARNING regarding risk of serious infections, and Medication Guide for additional information.
EMPAVELI was studied in two Phase 3 clinical trials for PNH:
Primary goal: to explore whether EMPAVELI was better at improving Hb levels than a C5i (eculizumab) in people living with PNH
Average change in Hb levels from beginning to Week 48
In the PEGASUS clinical trial, EMPAVELI was compared to the C5i treatment eculizumab.
The study consisted of 3 parts:
‡Noninferiority testsNoninferiority testsA test done to show that a treatment is not worse than another treatment. were used for these secondary goals. These tests determined if EMPAVELI was no worse than a C5i (eculizumab) compared to the beginning of the study.
“After 2 weeks into treatment, my Hb began to rise, and it has stayed consistently around 13. This is the type of response my doctor and I were hoping for.”
Emma is an adult with PNH who's taken EMPAVELI.
Individual experiences may vary.
At Week 16, EMPAVELI was no worse than a C5i (eculizumab) in helping people with PNH become transfusion-free.
of people taking EMPAVELI
were transfusion-free
of people taking a C5i (eculizumab)
were transfusion-free
At Week 16, EMPAVELI was no worse than a C5i (eculizumab) in helping people with PNH reduce their absolute reticulocyte count (ARC).
At Week 16, it could not be determined if EMPAVELI was any worse than C5i (eculizumab) in reducing LDH.
This information is for observation only and no conclusions can be made on the effect of EMPAVELI on LDH levels.
Although it was not formally tested, people in the study were asked to report their changes in levels of fatigue at Week 16.
Measuring fatigue improvement
Fatigue was measured using the FACIT-Fatigue scale, a 13-question survey. In the survey, participants rated the level of impact they felt that their fatigue had on daily activities and function.
This information is for observation only and no conclusions can be made on the effect of EMPAVELI on fatigue. No comparisons can be made between those taking EMPAVELI and those taking eculizumab.
The scale ranges from 0 to 52. Higher scores mean less fatigue.
Report of fatigue may be an under- or overestimation since participants were aware of the treatment they were being given.
FACIT=Functional Assessment of Chronic Illness Therapy.
In a post hoc analysispost hoc analysisAn analysis that is done after a study has ended. It gathers additional findings based on what happened in the study. It is not considered as scientifically strong as an analysis that was planned before the study began. of PEGASUS data, most people taking EMPAVELI reported fatigue levels consistent with adults who don’t have PNH
A post hoc analysispost hoc analysisAn analysis that is done after a study has ended. It gathers additional findings based on what happened in the study. It is not considered as scientifically strong as an analysis that was planned before the study began. of PEGASUS data found a link between Hb and fatigue
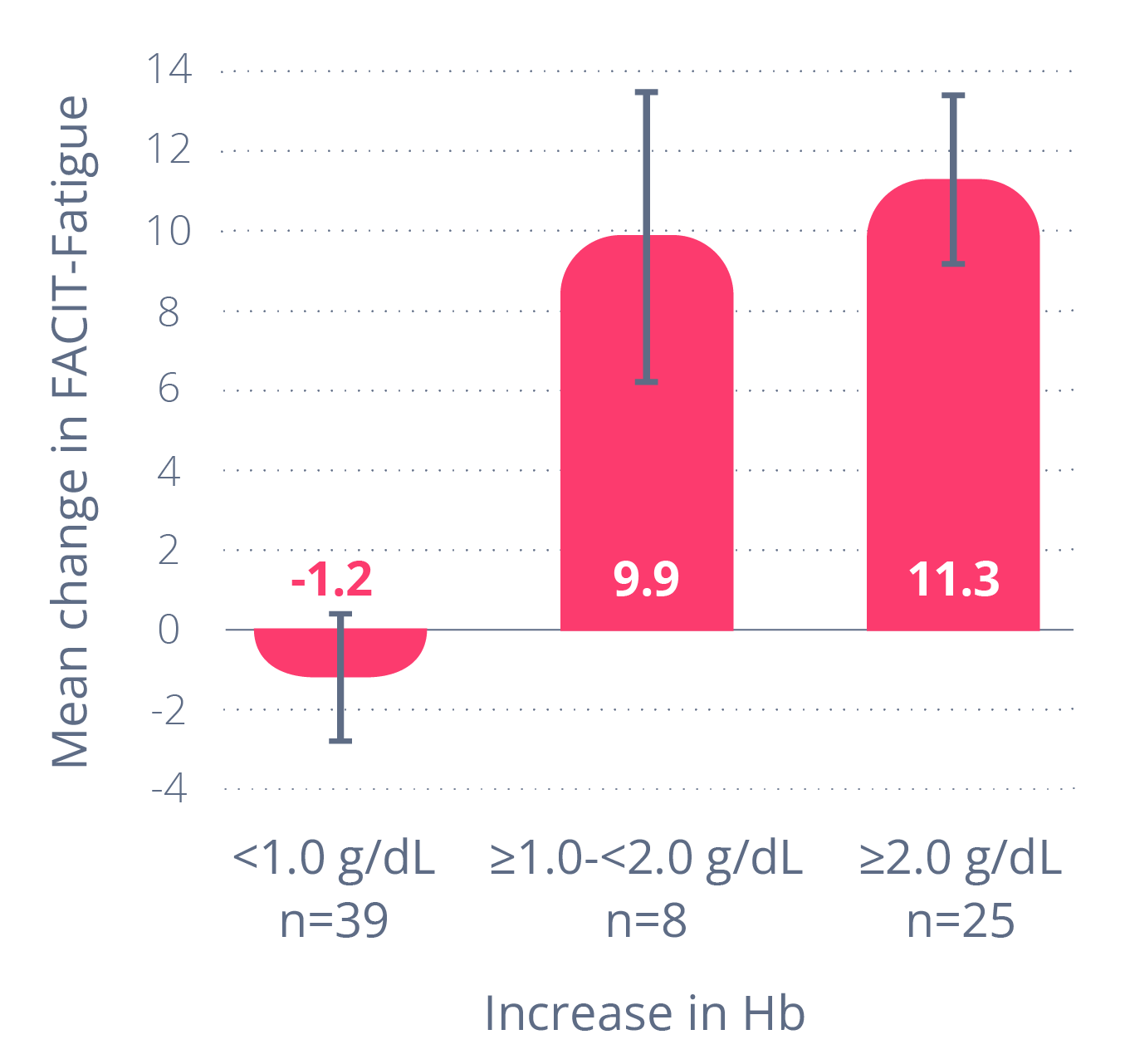
People with PNH who had an Hb improvement of 1 g/dL or more
had an average fatigue improvement of ~10 points.

“I love leisurely walks and vacations with my family. With my increase in Hb levels and energy, I don't have to miss out.”
Andrea is an adult with PNH who's taken EMPAVELI.
Individual experiences may vary.
What is normalization? How is it different than stabilization?
NormalizationNormalizationLab values improving to levels like those of adults who don’t have PNH. means lab values improved to levels like those of adults who don’t have PNH. This is different than stabilizationstabilizationLab values remaining at a stable level and not getting worse., which generally means that lab values did not get worse.
This information is for observation only and no conclusions can be made on the effect of EMPAVELI on Hb normalization. No comparisons can be made between those taking EMPAVELI and those taking eculizumab.
At Week 16 in PEGASUS
34%
of people taking
EMPAVELI achieved
Hb normalization
These data include only people who did not require a transfusion during the study.
Hb normalization means their Hb got to within the normal range (12-16 g/dL for females, 13.6-18 g/dL for males).
This information is for observation only and no conclusions can be made on the effect of EMPAVELI on LDH normalization. No comparisons can be made between those taking EMPAVELI and those taking eculizumab.
The majority of people taking EMPAVELI reduced their LDH levels to within the normal range (113-226 U/L).
At Week 16 in PEGASUS
71%
of people taking
EMPAVELI achieved
LDH normalization
These data include only people who did not require a transfusion during the study.
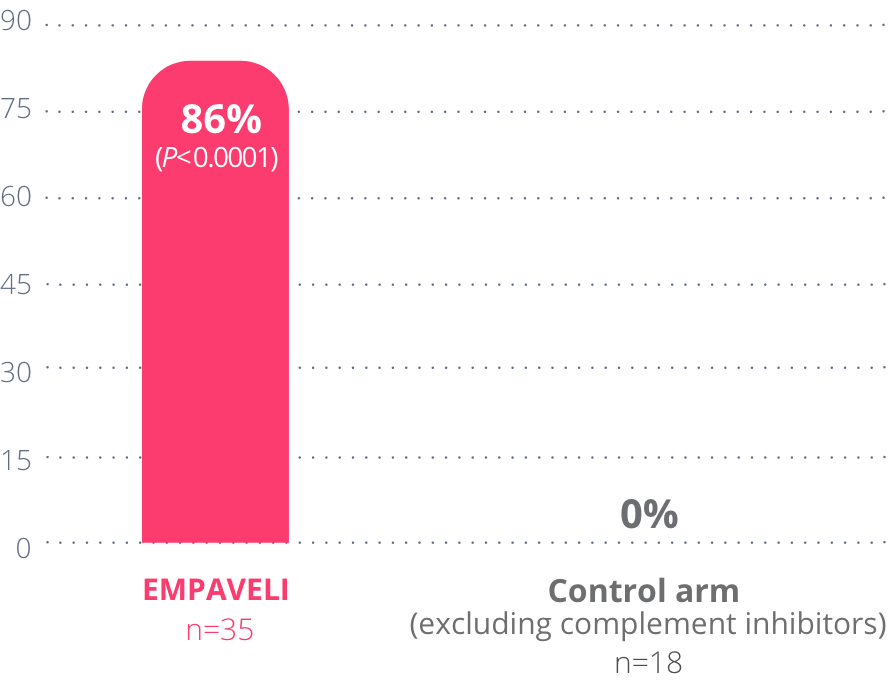
86%
of people taking
EMPAVELI achieved
Hb stabilization
over 26 weeks
*Hb stabilization means avoiding a >1 g/dL decrease in Hb levels throughout the study.
This information is for observation only and no conclusions can be made on the effect of EMPAVELI on Hb normalization. No comparisons can be made between those taking EMPAVELI and those in the control arm (excluding complement inhibitors).
At Week 26 in PRINCE
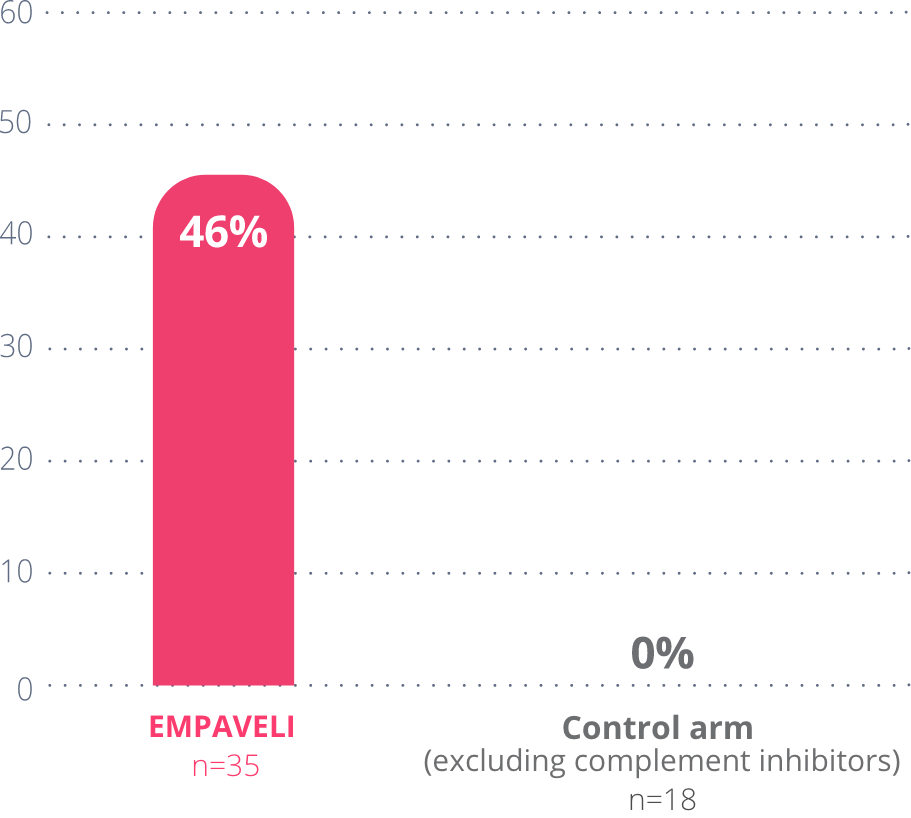
46%
of people taking
EMPAVELI achieved
Hb normalization
These data include only people who did not require a transfusion during the study.
Hb normalization means their Hb got to within the normal range (12-16 g/dL for females, 13.6-18 g/dL for males).
Rapid and sustained LDH reductions
By Week 2 in PRINCE, average LDH levels in the EMPAVELI group had dropped from baseline and remained there through 26 weeks.
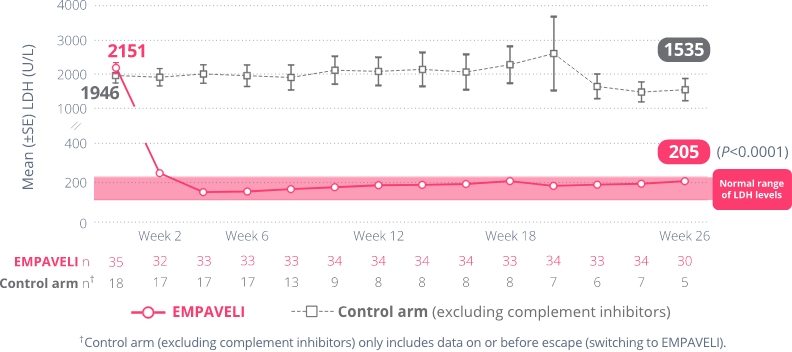
At Week 26, average reductions in LDH levels were:
This information is for observation only and no conclusions can be made on the effect of EMPAVELI on LDH normalization. No comparisons can be made between those taking EMPAVELI and those in the control arm (excluding complement inhibitors).
Nearly 2/3 of people taking EMPAVELI reduced their LDH levels to within the normal range (113-226 U/L).
At Week 26 in PRINCE
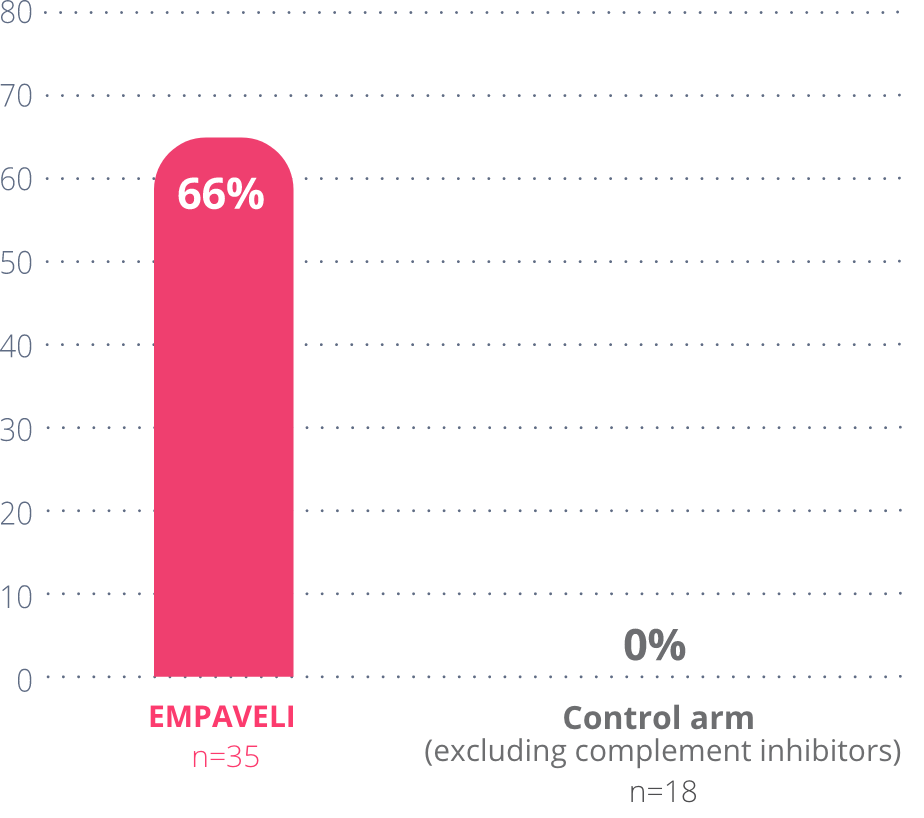
66%
of people taking
EMPAVELI achieved
LDH normalization
These data include only people who did not require a transfusion during the study.
At Week 26, most people taking EMPAVELI were transfusion-free.
of people taking EMPAVELI
were transfusion-free
of people in the
control arm (excluding
complement inhibitors)
were transfusion-free
The PRINCE clinical trial compared people taking EMPAVELI to people in a control arm, which meant they were not taking EMPAVELI or any type of complement inhibitor treatment. People in the control arm continued their non–complement inhibitor medications, if they were taking any.
An open-label extension study included adults with PNH (N=114) who enrolled and had previously completed the EMPAVELI Phase 3 studies (PEGASUS, PRINCE). Long-term data presented are from an integrated analysis of data in the subset of people who received 1080 mg of EMPAVELI by subcutaneous infusion twice weekly, or every 3 days, for up to 3 years (2.5 years, PRINCE; 3 years, PEGASUS).
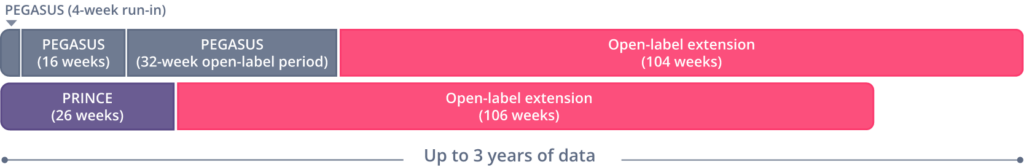
The goal of this analysis was to evaluate the long-term effectiveness and safety of EMPAVELI in treating adults with PNH.
Hb levels up to 3 years
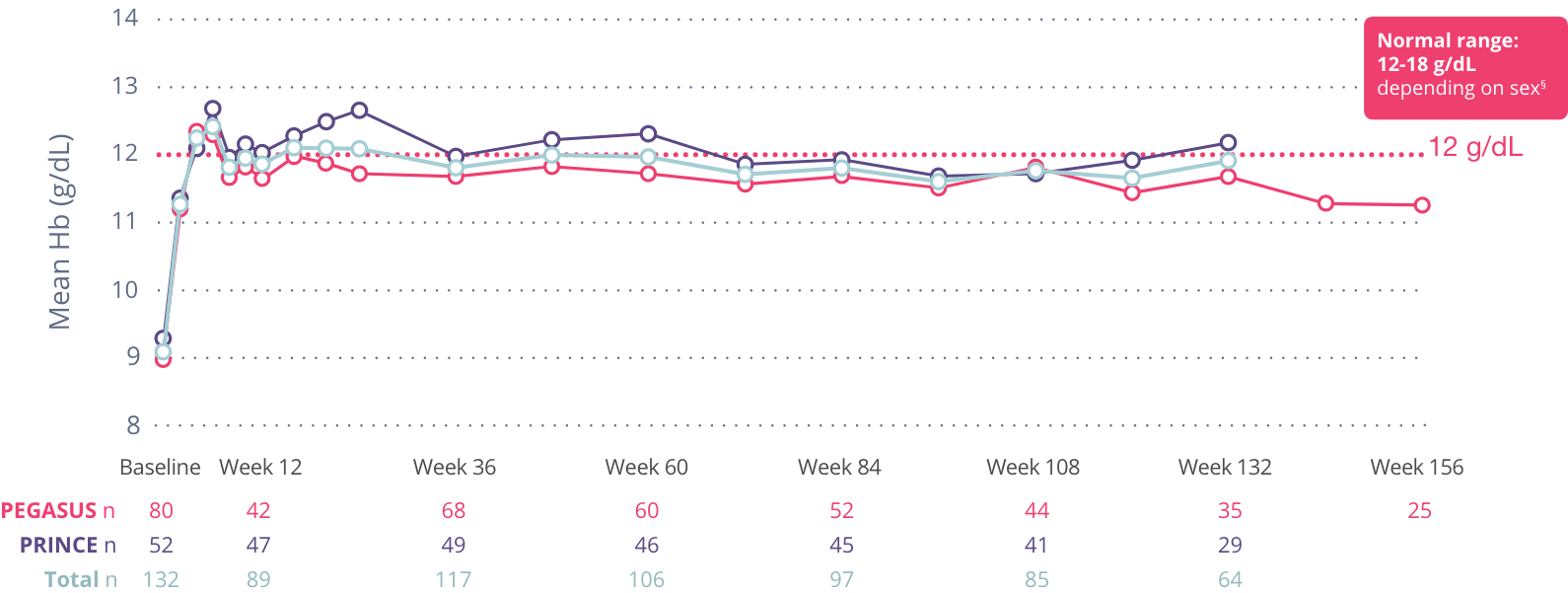
Patients who had a transfusion were not included in the Hb analysis for 60 days after the transfusion.
§12-16 g/dL for females, 13.6-18 g/dL for males.
LDH levels up to 3 years
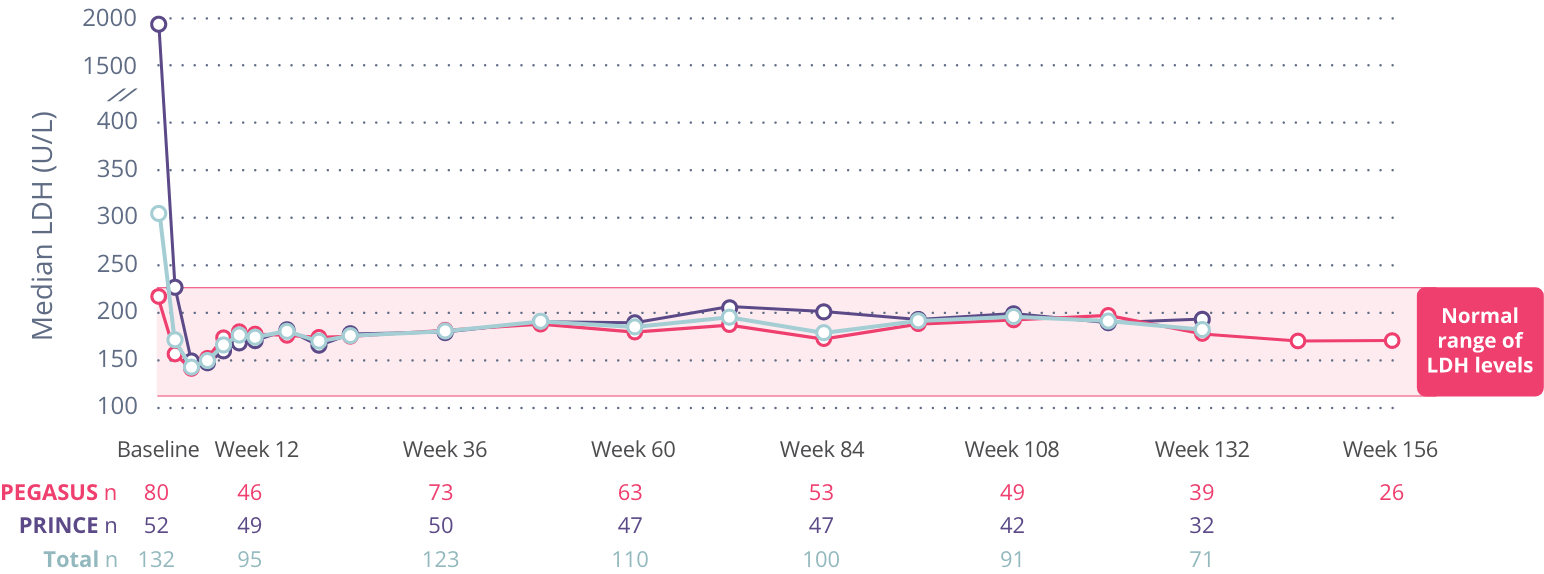
Overall, no new or unexpected side effects were identified.

“Before EMPAVELI, my Hb and LDH were not where my doctor and I wanted them to be. Now they’re at comfortably normal levels.”
Louis is an adult with PNH who's taken EMPAVELI.
Individual experiences may vary.
EMPAVELI is a medicine that affects your immune system and may lower the ability of your immune system to fight infections.
EMPAVELI increases your chance of getting serious infections caused by encapsulated bacteria such as Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B. These serious infections may quickly become life-threatening or cause death if not recognized and treated early.
Your healthcare provider will give you a Patient Safety Card about the risk of serious infections. Carry it with you at all times during treatment and for 2 months after your last EMPAVELI dose. Your risk of serious infections may continue for several weeks after your last dose of EMPAVELI. It is important to show this card to any healthcare provider who treats you. This will help them diagnose and treat you quickly.
EMPAVELI is only available through a program called the EMPAVELI Risk Evaluation and Mitigation Strategy (REMS). Before you can take EMPAVELI, your healthcare provider must enroll in the EMPAVELI REMS program, counsel you about the risk of serious infections caused by certain bacteria, give you information about the symptoms of serious infections, make sure that you are vaccinated against serious infections caused by encapsulated bacteria and that you receive antibiotics if you need to start EMPAVELI right away and you are not up to date on your vaccines, and give you a Patient Safety Card about your risk of serious infections.
Do not take EMPAVELI if you:
Before you take EMPAVELI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the vaccines you receive and medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements which could affect your treatment.
If you stop taking EMPAVELI, your healthcare provider will need to monitor you closely for at least 8 weeks after stopping EMPAVELI. Stopping treatment with EMPAVELI may cause a breakdown of red blood cells due to PNH.
Symptoms or problems that can happen due to red blood cell breakdown include:
EMPAVELI can cause serious side effects including allergic reactions. Allergic reactions can happen during your EMPAVELI infusion. Stop your EMPAVELI infusion and tell your healthcare provider or get emergency medical care right away if you get any of these symptoms during your EMPAVELI infusion:
The most common side effects in people with PNH treated with EMPAVELI include injection-site reactions; infections; diarrhea; pain in the stomach (abdomen); respiratory tract infection; pain in the arms, hands, legs, or feet; low potassium in blood; tiredness; viral infection; cough; joint pain; dizziness; headache; and rash.
These are not all of the possible side effects of EMPAVELI. Tell your healthcare provider about any side effect that bothers you or that does not go away.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
EMPAVELI is a prescription medicine used to treat adults with a disease called paroxysmal nocturnal hemoglobinuria (PNH).
Please see full Prescribing Information, including Boxed WARNING regarding risk of serious infections, and Medication Guide for additional information.
Get the latest news on EMPAVELI sign up now

The information on this website is intended for US healthcare providers.
By submitting this form, I acknowledge that I have reviewed, understood, and agree to the below guidelines, that I am at least 18 years of age and am legally competent to enter into this agreement, that I own all rights to the content submitted, and that I have received permission to submit the content from any other individual appearing in my submission for use by Apellis Pharmaceuticals, Inc. (“Apellis”), as set forth below.
Apellis can take, collect and use any content I have submitted to this webpage, including my photographs, film or recordings taken of my image and voice, my quotation or testimonial, as well as any personal health information contained in such photographs, film or recordings or provided on this submission form (collectively, “My Content and Information”). Apellis reserves the right to not post or use My Content and Information.
Apellis and its subsidiaries, affiliates, licensees, successors, assignees and those acting with its authority, have the right to use, re-use, broadcast, display and publish My Content and Information on social media, websites, displays, and emails. I waive all rights that I may have to claims for payment or royalties in connection with the posting or other usage of My Content and Information on these platforms. Apellis will contact me by e-mail or phone if it would like to feature My Content and Information elsewhere.
Any content that I submit to Apellis may be edited or excerpted in Apellis’ sole discretion to align with promotional requirements and the intended use of this site. I waive all rights in My Content and Information, including any right to inspect or approve My Content and Information prior to use or dissemination by Apellis. If significant changes are required, Apellis may contact me via e-mail or phone. Apellis will not use my e-mail address or phone number for any other purpose, unless I have separately opted into receiving communications from Apellis.
Adverse Events (“AEs”): Apellis will address any adverse events or side effects mentioned within submissions according to FDA regulations.
You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.