Take charge with
twice-weekly dosing and
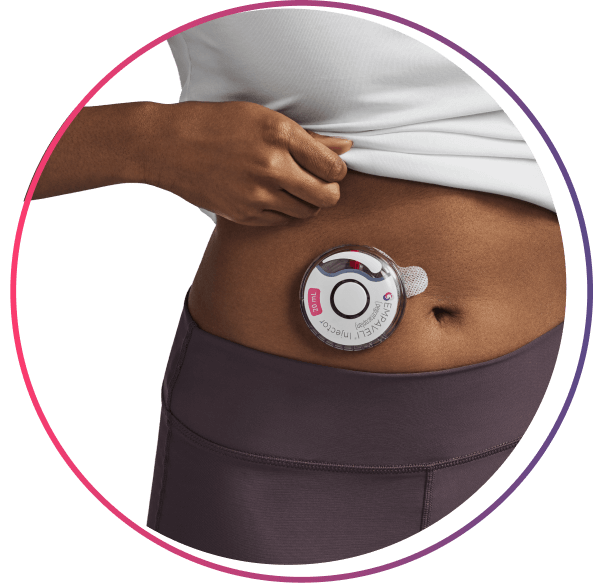
the EMPAVELI Injector
Laura is an adult with PNH who’s taken EMPAVELI
The EMPAVELI injector works for your schedule








>97% compliance* reported with at-home administration
Nearly 100% of patients reported they were confident self-administering EMPAVELI after receiving training from an Apellis Care Educator†
*Compliance calculated by medical possession ratio of >350 US patients on EMPAVELI. Data as of 03/31/2025.
†Based on feedback from >300 people with PNH after receiving self-administration training with an Apellis Care Educator. They rated their confidence with self-infusion on a scale from 1-7. A score of ≥5 was considered “confident.” Data as of 05/21/2025.
See how to use EMPAVELI

“Being able to self-administer EMPAVELI is a huge improvement over going to the infusion center. It’s more convenient, and it’s also easier not being in a clinical setting. And the in-home training from the ACE was incredibly helpful!”
Keep in mind while taking EMPAVELI:
Avoid intense physical activity and do not bump or knock the EMPAVELI Injector or button during the injection. Keep the skin on your abdomen completely dry.
EMPAVELI is designed to stay in your system but if you miss a dose, take your missed dose as soon as possible and resume the regular dosing schedule.
You will be trained before using the EMPAVELI Injector for the first time. See the EMPAVELI Injector Instructions for Use or, if using an infusion pump, see those specific Instructions for Use.
Jesus is an adult with PNH who’s taken EMPAVELI
Actor portrayal.
What else should I know before starting EMPAVELI?
EMPAVELI REMS program ensures your safety is a top priority
Because of the risk of serious infections caused by encapsulated bacteria, EMPAVELI is only available through a program called the EMPAVELI Risk Evaluation and Mitigation Strategy (REMS).
REMS is a safety program run by the FDA. Before you can take EMPAVELI, your doctor must enroll in the program and will provide you with the following:
- Counseling on the risk of serious infections caused by certain bacteria
- Information about the symptoms of serious infections
- Appropriate vaccinations against serious infections caused by encapsulated bacteria
- You will receive antibiotics if you need to start EMPAVELI right away and are not up to date on your vaccines.
Find vaccine support >
- You will receive antibiotics if you need to start EMPAVELI right away and are not up to date on your vaccines.
- A Patient Safety Card
- Carry this card with you at all times during treatment and for 2 months after your last EMPAVELI dose
- Show this card to any healthcare professional to help diagnose and treat you quickly
- Your risk of serious infection may continue for several weeks after your last dose of EMPAVELI
Certain vaccines are required before starting EMPAVELI
At least 2 weeks before your first dose of EMPAVELI, complete or update your vaccinations against Streptococcus pneumoniae and Neisseria meningitidis.
You may need the following vaccines:
- Pneumococcal vaccination(s)
- Meningococcal vaccinations
- MenACWY series
- MenB series
To see where you can get vaccinated, start by talking to your doctor to see if they offer the vaccines.
Vaccines may also be available at:
- Retail pharmacies
- Health clinics
- Your local health department
As part of the ApellisAssist program, a Vaccine Coordinator can help you with this process.
Reach out to your doctor if you have any additional questions about starting EMPAVELI.
Your doctor will provide guidance on how to switch from your previous treatment to EMPAVELI in order to provide a smooth transition and reduce the risk of hemolysis from treatment discontinuation. That guidance may include the following:
- If you are switching from eculizumab, you should start EMPAVELI while on your current dose of eculizumab. After 4 weeks, you may discontinue eculizumab and continue to take EMPAVELI on its own
- If you are switching from ravulizumab, you should start EMPAVELI no more than 4 weeks after your last dose of ravulizumab
With the ApellisAssist patient support program, you will have the help of an Apellis Care Educator (ACE). ACEs have nursing backgrounds and will provide you with self-administration training.
They will continue to be by your side throughout your journey with ongoing support, education, and answers to your questions.
ACEs do not give medical advice. Talk to your doctor for treatment-related questions.

“My ACE is amazing. Her training helped give me confidence in self-administering EMPAVELI. She continues to be there for me and is always a phone call away.”
ACE=Apellis Care Educator; PNH=paroxysmal nocturnal hemoglobinuria.
